
CLOSURE-AF-DZHK16
Left atrial appendage CLOSURE in patients with Atrial Fibrillation at high risk of stroke and bleeding compared to medical therapy: a prospective randomized clinical trial
STUDY DESIGN
Prospective, multi-centre, national, unblinded, randomized study.
Study objective
Study goal is to determine the clinical benefit of a strategy of percutaneous catheter‐based left atrial appendage (LAA) closure in patients with non-valvular atrial fibrillation (NVAF) at high risk of stroke (CHA2DS2‐VASc Score ≥2) and bleeding as compared to best medical care.
Description
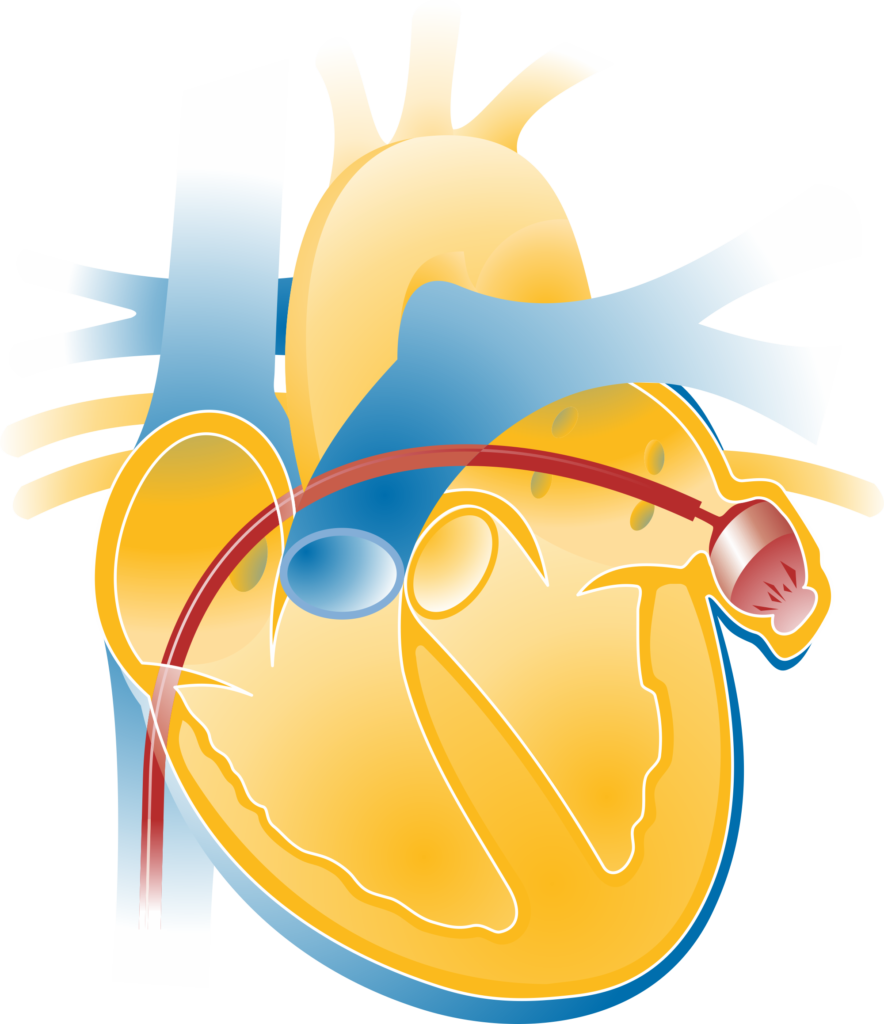
Atrial fibrillation is the most common cardiac arrhythmia in Germany. With atrial fibrillation, there is a risk of clots forming in the heart, particularly in the left atrial appendage), a part of the heart. If these clots come loose, there is a risk of stroke. To prevent strokes, patients with atrial fibrillation can be given medication known as anticoagulants. These drugs prevent the formation of clots in the heart.
Another treatment option is to close the left atrial appendage with a closure system (occluder), which prevents clots from reaching the brain or the body. After the left atrial appendage has been closed, the blood only needs to be diluted for a short time until the occluder has healed. The CLOSURE-AF study compares the two clinically established treatment approaches (blood thinning with medication and closure of the left heart ear using a catheter technique). Only approved anticoagulants and closure systems will be used.
CONTACT INFORMATION

Prof. Dr. med. Ulf Landmesser
Director of Studies
Charité – Universitätsmedizin Berlin

Prof. Dr. med. Ingo Eitel
Co-study director
Medizinische Klinik II, Universitäres Herzzentrum Lübeck
Universitätsklinikum Schleswig-Holstein

Prof. Dr. med. Leif-Hendrick Boldt
Co-study director
Medizinische Klinik mit Schwerpunkt Kardiologie
Charité – Universitätsmedizin Berlin
FURTHER INFORMATION
AFNET ROLE
Regulatory Project Management, organisation and support of committees (CEC) and meetings, support of the study coordinator with e.g. supervision of sites